During the 72nd Regional Committee (RC) meeting in September 2019, the WHO South-East Asia (SEA) regional goal was revised to measles and rubella elimination by 2023. The measles and rubella (MR) laboratory network in the SEA Region was established in 2003 with eight laboratories. It has since then been expanded to 46 Proficient laboratories with plans for inclusion of another 10 laboratories to the network.
The SEAR Immunization Technical Advisory Group (ITAG) recommended that the Region should develop a laboratory quality management system (LQMS) to ensure sustained proficiency of the measles rubella laboratory network.
The Scope of this e-learning is based on the LQMS plan to provide a step-wise process for performing internal and external audits by using the most current WHO accreditation checklists and providing critical points for reviewing the testing performance of IgM detection, molecular testing and virus isolation. The audits will look into the whole process of testing: pre-analysis, analysis and post-analysis.
Язык: English
Measles Rubella
Информация о курсе
Overview : This course provides a step-wise process for performing internal and external audits by using the most current WHO accreditation checklists and providing critical points for reviewing the testing performance of IgM detection, molecular testing and virus isolation. The audits will look into the whole process of testing: pre-analysis, analysis and post-analysis.

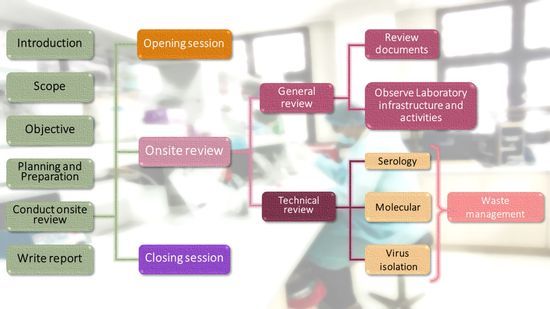
- This course comprises 7 modules:
- Module 1: Introduction
- Module 2: Conduct onsite review as part of opening, closing session and writing report.
- Module 3: Onsite review as part of general review
- Module 4: Onsite review as part of serology, IgM detection by ELISA
- Module 5: Onsite review as part of molecular testing including
5.1 Real-time RT-PCR
5.2 End-point RT-PCR
5.3 Sequencing
5.4 Genotyping
- Module 6: Onsite review as part of virus isolation
- Module 7: Onsite review as part of waste management
- Please noted :
1) Participants should study modules 1 to 3 prior to learning modules 4 to 7
2) Module 4 to 7 can be learnt independently.
3) There is post-test after learning of each module 4, 5, 6 and 7.
- Learning objectives : By the end of this course, participants would be able to:
• Perform laboratory accreditation review of measles and rubella IgM detection by ELISA;
• Perform laboratory accreditation review of measles and rubella virus detection by real-time RT-PCR and end-point RT-PCR;
• Perform laboratory accreditation review of measles and rubella virus sequencing and genotyping;
• Perform laboratory accreditation review of measles and rubella virus isolation;
• Define type of waste and components of an infectious waste management plan as well as reviewing appropriate on-site waste treatment.
Course duration : Approximately 1 hour and 40 mins.
Certificates : A Record of Achievement certificate will be available to participants who score at least 80% of the total points available across all of the quizzes. Participants who receive a Record of Achievement can also download an Open Badge for this course. Click here to learn how.
Записаться на этот курс
Требования для получения сертификата
- Чтобы получить сертификат об окончании курса, участникам необходимо набрать не менее 80% от максимального количества баллов за все задания на оценку.
- Gain an Open Badge by completing the course.